
FAERS: FDA Adverse Event Reporting System
The FDA Adverse Event Reporting System (FAERS) is a public database containing information on adverse events and medication errors. An adverse event is any symptom ...
Read more
The FDA Adverse Event Reporting System (FAERS) is a public database containing information on adverse events and medication errors. An adverse event is any symptom ...
Read more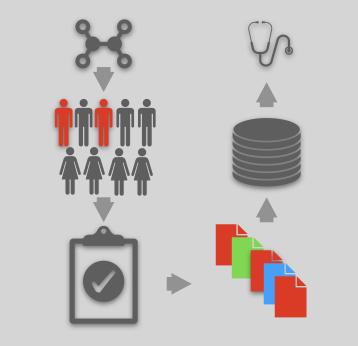
It is nearly impossible to design a drug molecule that only has targetted effect in all living individuals. Even commonly used over-the-counter drugs, deemed and recognized by authorities as safe, can cause undesirable effects in some "hypersensitive" individuals.
Read more
How does genetic make-up determine the possibility of developing ADRs to medications?
Read more