Three types of resources with evidence and actionable knowledge for evidence-based medicine
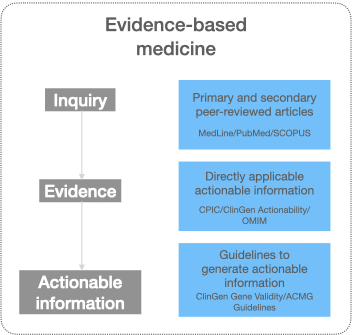
Evidence-based medicine (EBM) is the conscientious, explicit, judicious, and reasonable use of the latest evidence in making decisions for the care of patients. Depending on the specialty, knowingly or unknowingly, health care practitioners use evidence-based medicine to provide patient care. However, it is nearly impossible to stay up-to-date on the ever-growing medical knowledge where facts of today are updated or become obsolete in the near future. Physicians typically look for further evidence when they encounter a patient with a common disease but complex history, a rare disease, or emerging conditions like a pandemic. With such an encounter, the physician raises an inquiry, gathers evidence, weighs them, and arrives at actionable insights.
Although there are several resources available to find evidence and/or actionable knowledge, broadly, they belong to one of the three categories:
Resources with primary and secondary peer-reviewed articles
Journal articles describing original research in basic and medical sciences typically constitute primary sources of evidence. Generally, such reports provide a single evidence of association, indication, or contra-indication. Although of considerable value, reading an original research article requires domain-specific knowledge to understand the intricacies and validity of the study. Reviews, meta-analyses, clinical care notes constitute a secondary source of evidence. These articles summarize, compare, and contrast primary research articles to conclude. Based on the scope of the inquiry and status of the research in the field, physicians or working groups may focus on either primary or secondary articles. PubMed/MedLine constitutes a significant source to find and investigate primary and secondary articles to gather evidence or actionable knowledge.
Actionable knowledge published by authoritative sources
Authoritative, national, or international consortiums, non-profit organizations composed of prominent personalities in various fields collaboratively generate actionable knowledge. As a group, such organizations following agreed-upon protocol or guidelines develop a knowledge base to cater to general needs. One such example is the efforts of the Clinical Pharmacogenetics Implementation Consortium (CPIC). The primary objective of CPIC is to help clinics implement pharmacogenetics testing and translate the results into actionable decisions. With CPIC generated evidence and actionable knowledge, the physician can decide the type and dosage of a drug based on pharmacogenetics test results. CPIC makes the actionable knowledge publicly accessible on its website. Although this actionable knowledge is precious, as a single organization with limited people and informatics infrastructure, the throughput of curation is low. Another example of such efforts is the clinical actionability of genetic diseases published by the clinical genome resource (ClinGen) project. OMIM, Liver Tox are examples of resources with semi-actionable gene-disease and drug-induced liver toxicity information. A more scalable approach will be making the guidelines semi-quantitative and well-documented for broader adoption to generate actionable knowledge, as described in the next section.
Guideline based distributed actionable knowledge
Instead of directly generating actionable information, authoritative agencies often publish detailed guidelines on collecting, combining, weighing the evidence, and developing actionable knowledge to tailor patient care. Using this authoritative guideline, independent working groups in public or private sectors can generate and transparently communicate evidence, their strength, and actionable evidence (in peer-reviewed articles or on the world wide web). A remarkable example of such guideline is gene-disease validity guidelines published by the clinical genome resource ClinGen. In addition to ClinGen producing transparent, actionable information about the gene-disease association, the guidelines is used by various public and private agencies to weigh the evidence and modify test panels or offer patient care accordingly. Although this approach is scalable, the quality of data varies based on different geographical sites. Additionally, the end-user has to search multiple sites to find actionable knowledge.
Conclusion
There are different ways evidence and actionable information are directly or indirectly applicable to physicians for patient care; the quality and reliability vary. Federal agencies like the FDA have recently started evaluating and formally endorsing a database with valuable and actionable information in patient care. With more efforts toward actionable knowledge generation, the dream of personalized medicine will soon be a reality for almost every aspect of patient care.